 Fonctionnement - Solar fuel cell
Fonctionnement - Solar fuel cell
1. Electrolysis (separation of water into hydrogen and oxygen)
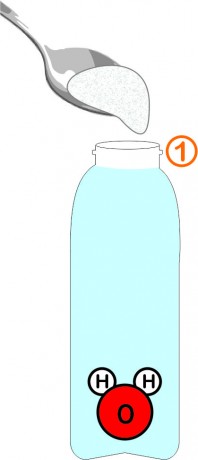
Fill the bottle with water, leaving about 2cm of air at the top. Add 1 large tablespoon of fine salt and mix until dissolved.
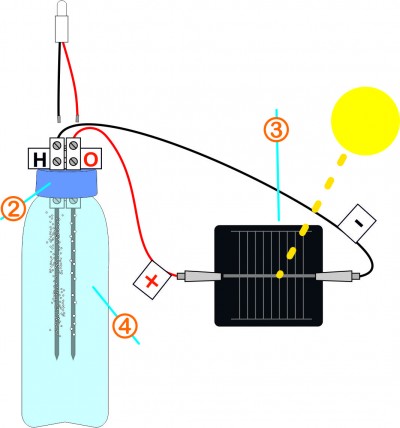
Very gently, place back the two graphite leads and screw the cap. Using crocodile clips, connect the contacts of the solar cell respecting the polarities.
Expose the cell to direct sunlight. To check if the electricity circulates throughout the circuit, touch the contacts of the connector with the end of the LED wires respecting the polarities. The LED should light up.
On the negative side, a large number of small bubbles will escape, it is hydrogen. On the positive side, larger bubbles less numerous will form and often stick to the lead, it is oxygen.
The water molecule is composed 'like a mickey': 2 small atoms of hydrogen (H) and 1 large atom of oxygen (O).

2. The fuel cell (electricity production by recombination of hydrogen and oxygen)
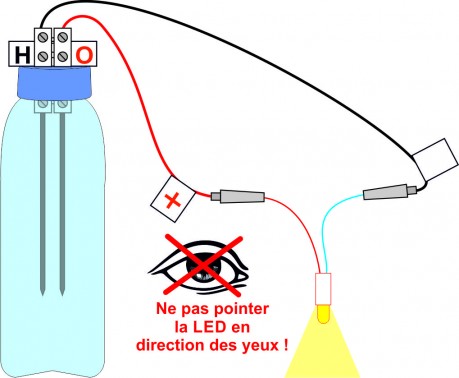
After an electrolysis of about 15 minutes, disconnect the cell and connect the LED instead respecting the polarities. The LED should light up for at least 15 to 30 seconds.
After several tests, the oxidation of the leads seems to improve the efficiency of the battery.
To check, time how long the LED works.
Try to modify some parameters to achieve the longest lighting time

Here is a short video that shows the production of hydrogen and oxygen from water.
 |  |  |  |  |  |  |


